Home /
Answered Questions /
Other /
use-the-following-data-to-determine-whether-the-conversion-of-diamond-to-graphite-is-exothermic-or-e-aw948
(Solved): Use The Following Data To Determine Whether The Conversion Of Diamond To Graphite Is Exothermic Or E...
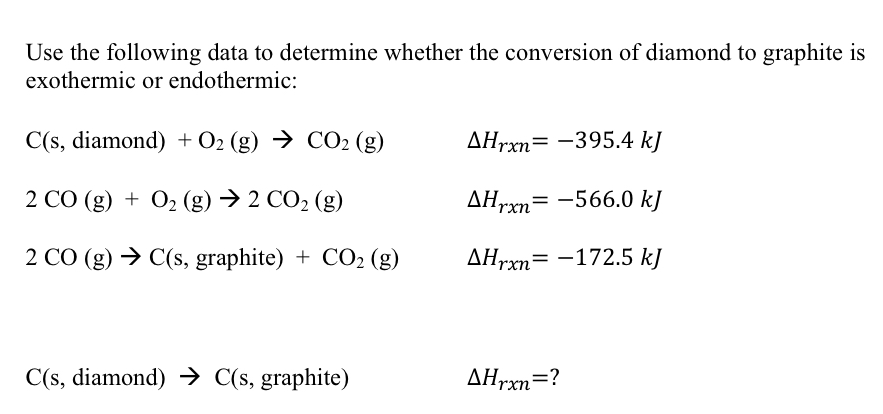
Use the following data to determine whether the conversion of diamond to graphite is exothermic or endothermic: C(s, diamond) + O2(g) → CO2 (g) AHrxn= -395.4 k] 2 CO (g) + O2 (g) → 2 CO2 (g) AHrxn= -566.0 kJ 2 CO (g) → C(s, graphite) + CO2 (g) Hrxn= -172.5 kJ C(s, diamond) → C(s, graphite) AHrxn=?
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
