Home /
Answered Questions /
Other /
the-reaction-c2h3no2-g-c2h4-g-hno2-g-is-first-order-in-an-experiment-the-rate-constant-is-determined-aw883
(Solved): The Reaction C2H3NO2(g) —+C2H4(g) +HNO2(g) Is First Order. In An Experiment, The Rate Constant Is ...
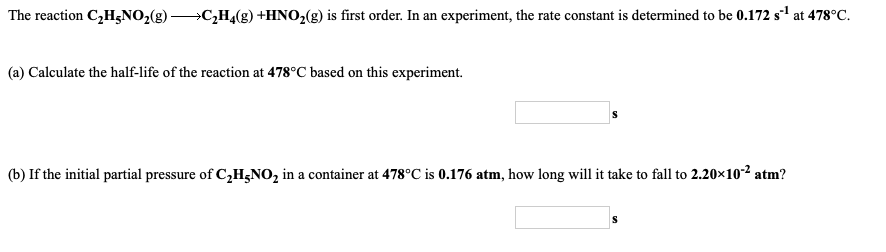
The reaction C2H3NO2(g) —+C2H4(g) +HNO2(g) is first order. In an experiment, the rate constant is determined to be 0.172 s1 at 478°C. (a) Calculate the half-life of the reaction at 478°C based on this experiment. (b) If the initial partial pressure of C2H NO2 in a container at 478°C is 0.176 atm, how long will it take to fall to 2.20x10-2 atm?
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
