Home /
Answered Questions /
Other /
the-rate-constant-of-the-elementary-reaction-c2h5no2-8-c2h4-8-hno2-g-is-k-6-39x10-35-1-at-401-c-and--aw888
(Solved): The Rate Constant Of The Elementary Reaction C2H5NO2(8) —*C2H4(8) +HNO2(g) Is K = 6.39x10-35-1 At ...
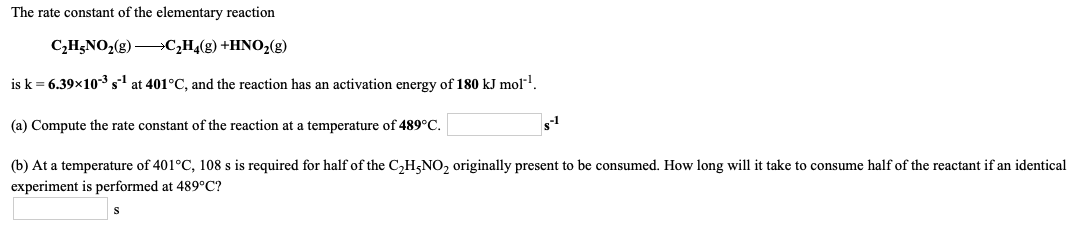
The rate constant of the elementary reaction C2H5NO2(8) —*C2H4(8) +HNO2(g) is k = 6.39x10-35-1 at 401°C, and the reaction has an activation energy of 180 kJ mol!. (a) Compute the rate constant of the reaction at a temperature of 489°C. (b) At a temperature of 401°C, 108 s is required for half of the C2H5NO2 originally present to be consumed. How long will it take to consume half of the reactant if an identical experiment is performed at 489°C?
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
