Home /
Answered Questions /
Other /
question-2-1-pts-look-at-sample-problem-18-8-write-a-balanced-chemical-equation-use-ha-for-the-acid--aw773
(Solved): Question 2 1 Pts Look At Sample Problem 18.8. Write A Balanced Chemical Equation (use HA For The Aci...
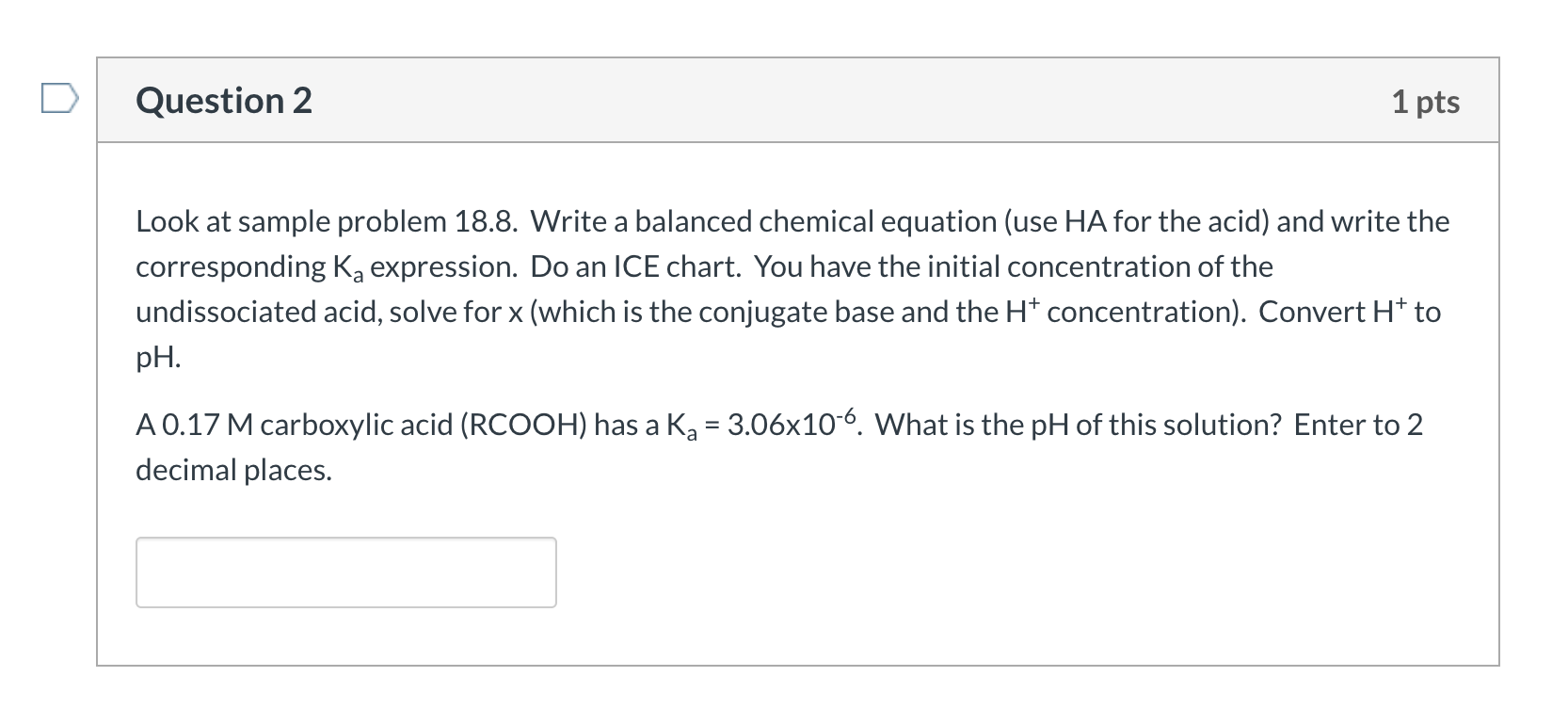
Question 2 1 pts Look at sample problem 18.8. Write a balanced chemical equation (use HA for the acid) and write the corresponding Ka expression. Do an ICE chart. You have the initial concentration of the undissociated acid, solve for x (which is the conjugate base and the H+ concentration). Convert Ht to pH. A 0.17 M carboxylic acid (RCOOH) has a Ka = 3.06x10-6. What is the pH of this solution? Enter to 2 decimal places.
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
