Home /
Answered Questions /
Other /
given-the-reaction-2-nahco3-s-na2co3-s-co2-g-h20-g-ah-f-kj-mol-s-imol-k-1-ag-f-kj-mol-nahco3-s-950-8-aw973
(Solved): Given The Reaction: 2 NaHCO3(s) = Na2CO3 (s) + CO2(g) + H20 (g) AH®f (kJ/mol) S°(Imol K-1 AG°f(kJ...
parts A-F
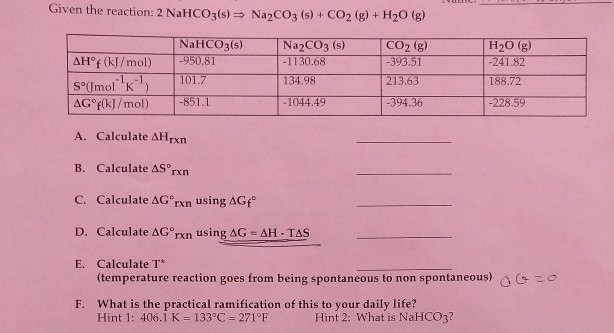
Given the reaction: 2 NaHCO3(s) = Na2CO3 (s) + CO2(g) + H20 (g) AH®f (kJ/mol) S°(Imol K-1 AG°f(kJ/mol) NaHCO3(s) -950.81 101.7 -851.1 Na2CO3(s) -1130.68 134.98 -1044.49 CO2 (g) -393.51 213.63 -394.36 H20 g -241.82 188.72 -228.59 A. Calculate AHrxn B. Calculate AS rxn C. Calculate AG®rxn using AGfº D. Calculate AGºrxn using AG = AH - TAS E. Calculate T* (temperature reaction goes from being spontaneous to non spontaneous) 20 F. What is the practical ramification of this to your daily life? Hint 1: 406.1 K = 133ºC = 271°F Hint 2: What is NaHCO3?
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
