Home /
Answered Questions /
Other /
cryolite-na-aif-s-an-ore-used-in-the-production-of-aluminum-can-be-synthesized-using-aluminum-oxide--aw159
(Solved): Cryolite, Na, AIF (s), An Ore Used In The Production Of Aluminum, Can Be Synthesized Using Aluminum ...
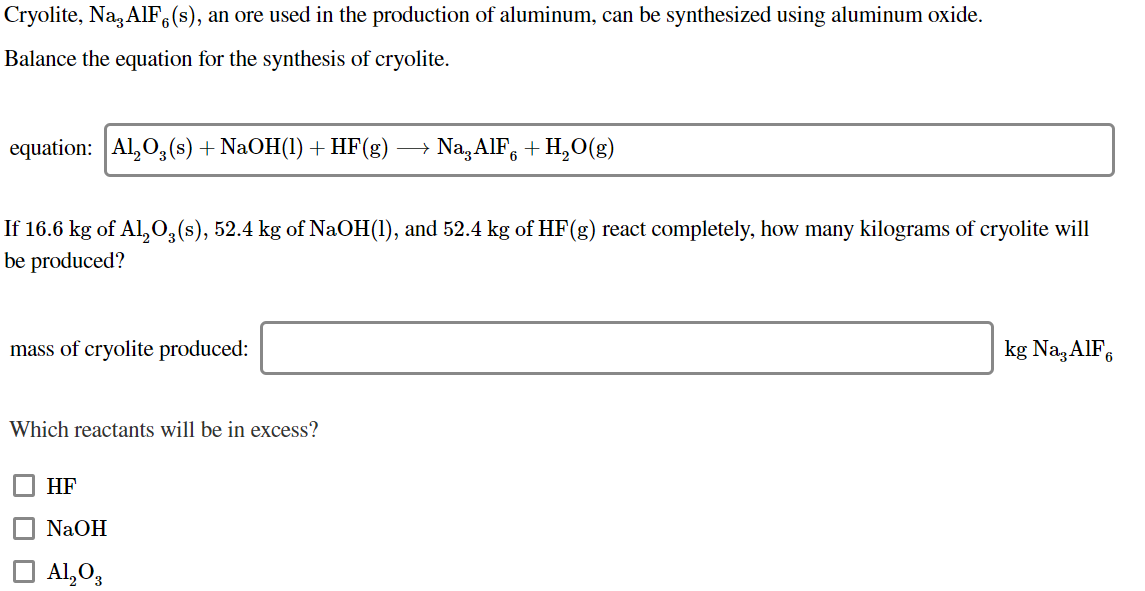

Cryolite, Na, AIF (s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. Balance the equation for the synthesis of cryolite. equation: Al2O3(s) + NaOH(1) + HF(g) + Na, A1F6 +H2O(g) If 16.6 kg of Al,O3(s), 52.4 kg of NaOH(1), and 52.4 kg of HF(g) react completely, how many kilograms of cryolite will be produced? mass of cryolite produced: kg Na3AlF6 Which reactants will be in excess? HF NaOH A1,0
What is the total mass of the excess reactants left over after the reaction is complete? total mass of excess reactants:
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
