Home /
Answered Questions /
Other /
calculate-the-vapor-pressure-at-25-c-of-a-solution-containing-55-8-g-ethylene-glycol-hoch-ch-oh-and--aw232
(Solved): Calculate The Vapor Pressure At 25°C Of A Solution Containing 55.8 G Ethylene Glycol (HOCH.CH,OH) A...
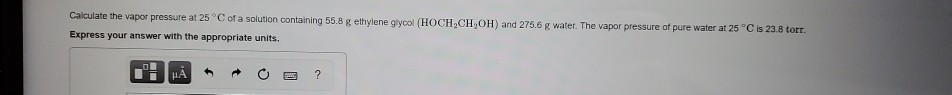
Calculate the vapor pressure at 25°C of a solution containing 55.8 g ethylene glycol (HOCH.CH,OH) and 275.6 g water. The vapor pressure of pure water at 25°C is 23.8 torr. Express your answer with the appropriate units. HA O ?
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
