Home /
Answered Questions /
Other /
alcohols-goals-build-molecular-models-of-alcohols-and-phenol-identify-physical-properties-of-alcohol-aw146
(Solved): ALCOHOLS Goals Build Molecular Models Of Alcohols And Phenol. • Identify Physical Properties Of Al...


help me answer questions 1-4 please.
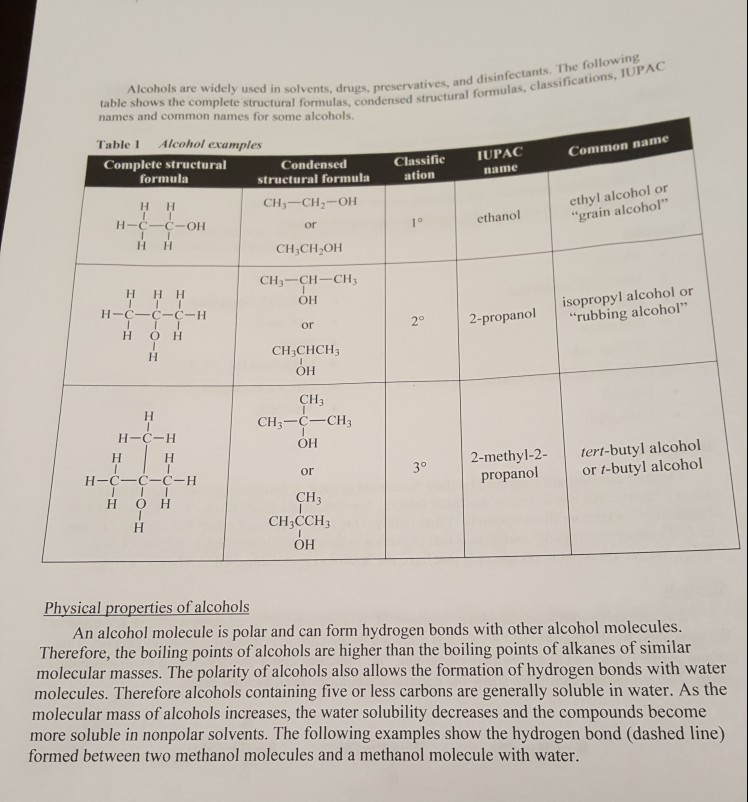
ALCOHOLS Goals Build molecular models of alcohols and phenol. • Identify physical properties of alcohols and phenol. • Use chemical tests to identify primary, secondary, and tertiary alcohols and phenol. • Use physical properties and chemical tests to identify an unknown. • Write balanced equations for oxidation reactions of alcohols. Materials • Gloves Molecular model kits Test tube rack Test tubes Stirring rod • pH paper and color charts • Ethanol • 1-Butanol 2-Butanol 2-Methyl-2-propanol 1-Octanol Phenol Unknowns (#1, #2, #3) • Chromate solution • 1% FeCl3 Discussion Organic families are classified by their functional groups. A functional group is an atom or group of atoms that has a certain structural feature. The functional group gives similar physical and chemical properties to all compounds in that family. One such functional group is a hydroxyl group, OH. Alcohols are organic compounds that contain a hydroxyl group attached to a hydrocarbon, while phenols are organic compounds that have the hydroxyl group attached to a benzene ring. Alcohols There are three classes of alcohols: primary (1º), secondary (2°), and tertiary (3%). Each class is distinguished by the number of "R" groups attached to the carbon bearing the hydroxyl group. This carbon is often referred to as the alcohol carbon. "R" indicates either an alkyl group or an aromatic group. The following examples show the general formula for each alcohol class. R-CH2-OH R-CH-OH R-C-OH primary alcohol secondary alcohol tertiary alcohol 10
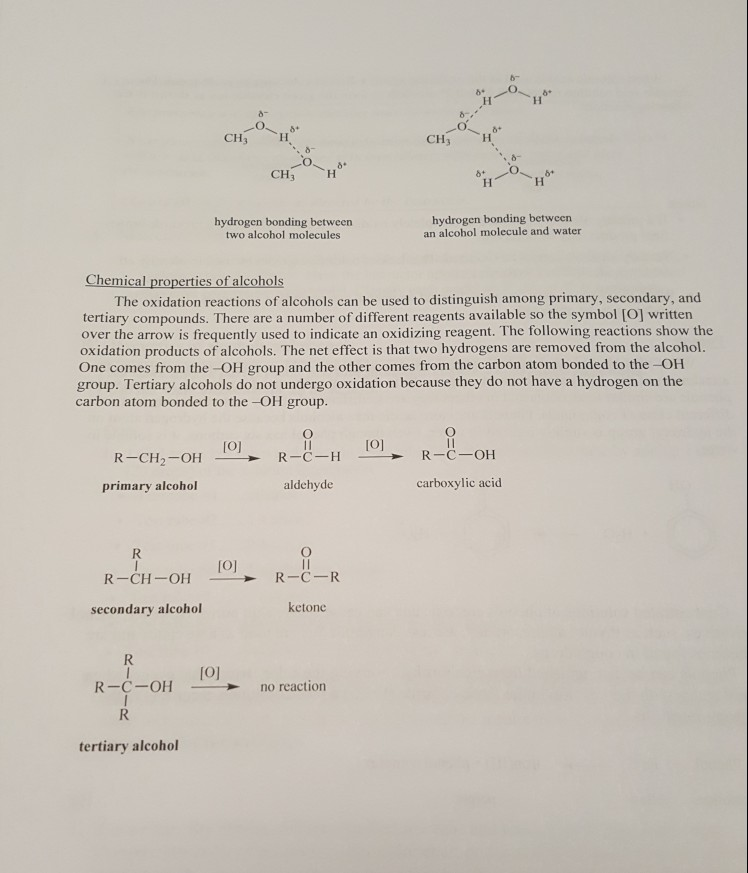
Alcohols are widely used in solvents, drugs, preservatives, and table shows the complete structural formulas, condensed structura names and common names for some alcohols. servatives, and disinfectants. The following structural formulas, classifications, IUPAC IUPAC Common name ation name Table 1 Alcohol examples Complete structural Condensed formula structural formula HH CH, -CH2-OH H-C-C-OH or HH CH,CH,OH ethyl alcohol or "grain alcohol" ethanol HHH H-C- C-C-H H oH CH3-CH-CH; ОРor CH3CHCH ОРisopropyl alcohol or "rubbing alcohol" 20 2-propanol CH3 CH3-C-CH ОРH-C-H 30 or tert-butyl alcohol or t-butyl alcohol 2-methyl-2- propanol H-C-C-C-H H OH CH3 CH3CCH ОРPhysical properties of alcohols An alcohol molecule is polar and can form hydrogen bonds with other alcohol molecules. Therefore, the boiling points of alcohols are higher than the boiling points of alkanes of similar molecular masses. The polarity of alcohols also allows the formation of hydrogen bonds with water molecules. Therefore alcohols containing five or less carbons are generally soluble in water. As the molecular mass of alcohols increases, the water solubility decreases and the compounds become more soluble in nonpolar solvents. The following examples show the hydrogen bond (dashed line) formed between two methanol molecules and a methanol molecule with water.
CH CHH CH CH** hydrogen bonding between two alcohol molecules hydrogen bonding between an alcohol molecule and water Chemical properties of alcohols The oxidation reactions of alcohols can be used to distinguish among primary, secondary, and tertiary compounds. There are a number of different reagents available so the symbol [O] written over the arrow is frequently used to indicate an oxidizing reagent. The following reactions show the oxidation products of alcohols. The net effect is that two hydrogens are removed from the alcohol. One comes from the OH group and the other comes from the carbon atom bonded to the OH group. Tertiary alcohols do not undergo oxidation because they do not have a hydrogen on the carbon atom bonded to the -OH group. fojio JOI R -C - H R-C-OH R-CH2-OH primary alcohol aldehyde carboxylic acid R R-CH-OH [O] - R-C-R secondary alcohol ketone JOJ R-C-OH no reaction tertiary alcohol
Introduction to Organic Chemistry When chromic acid is used as the oxidizing agent, a distinct color change is observed. The chromic acid solution reacts with 1° and 2º alcohols to form the green chromic ion as shown in the following reaction: 1° or 2" alcohol + Cr20,2 oxidized alcohol product + Cr dichromate ion chromic ion present in chromic acid (green) (orange) Notes: • Ir a primary alcohol is allowed to completely oxidize to a carboxylic acid, a greenish-brown final product may be observed. • Tertiary alcohols cannot be oxidized. This lack of color change can be used to identify an unknown alcohol as a tertiary alcohol • When the chromic acid test is performed on phenol compounds, a brownish-black tar-like product may be observed. Phenols While phenols and alcohols both have the hydroxyl group (OH) as their functional group, the attachment of the -OH onto a benzene ring results in different properties for phenols. In some ways, phenols are similar to alcohols and in others they are so different that they seem to belong to a different class of compounds. Phenols are more acidic than alcohols because the hydrogen atom on the hydroxyl group is slightly ionized in water. Even though phenol has six carbons, it is soluble in water. ОР+ H2O + H20 Concentrated solutions of phenols are toxic and can cause severe skin burns. However, phenol derivatives, such as thymol and resorcinol, are less dangerous and are used as antiseptics and are sometimes found in cough drops. Phenols can be distinguished from alcohols by observing the color change that occurs when phenol reacts with the Fe" ion in the ferric chloride (FeCl3) aqueous solution according to the following reaction: Phenol + Fe3+ - iron(III) . phenol complex colorless yellow purple
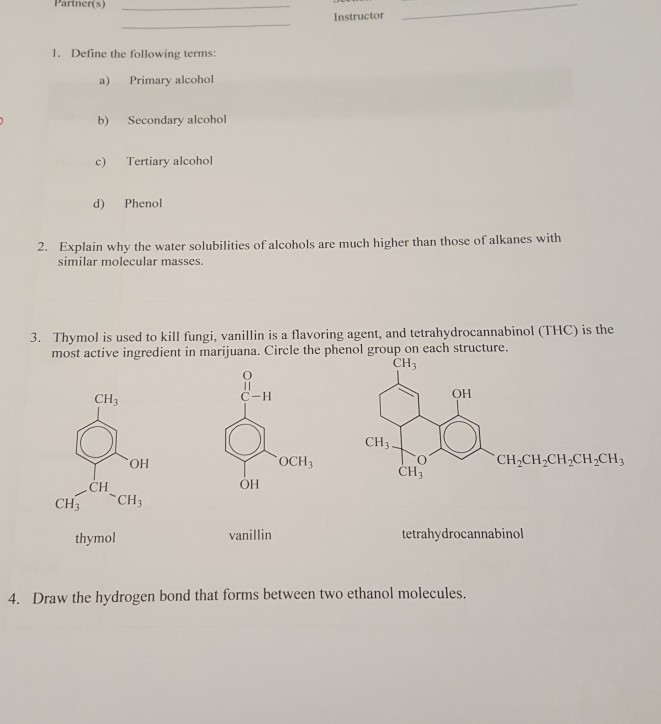
Instructor 1. Define the following terms: a) Primary alcohol b) Secondary alcohol c) Tertiary alcohol d) Phenol 2. Explain why the water solubilities of alcohols are much higher than those of alkanes with similar molecular masses. 3. Thymol is used to kill fungi, vanillin is a flavoring agent, and tetrahydrocannabinol (THC) is the most active ingredient in marijuana. Circle the phenol group on each structure. CH C-H T oCH, CH,CH CH CH.CH CH ТОРCH CH3 CH3 OH thymol vanillin tetrahydrocannabinol 4. Draw the hydrogen bond that forms between two ethanol molecules.
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
