Home /
Answered Questions /
Other /
when-0-424-g-of-biphenyl-c12h10-undergoes-combustion-in-a-bomb-calorimeter-the-temperature-rises-fro-aw760
(Solved): When 0.424 G Of Biphenyl (C12H10) Undergoes Combustion In A Bomb Calorimeter, The Temperature Rises ...
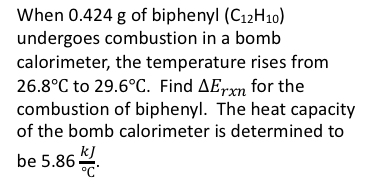
When 0.424 g of biphenyl (C12H10) undergoes combustion in a bomb calorimeter, the temperature rises from 26.8°C to 29.6°C. Find AErxn for the combustion of biphenyl. The heat capacity of the bomb calorimeter is determined to be 5.86
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
