Home /
Answered Questions /
Other /
please-help-me-about-calculation-the-limiting-reagent-and-theoritical-yield-of-this-reaction-with-gr-aw484
(Solved): Please Help Me About Calculation The Limiting Reagent And Theoritical Yield Of This Reaction With Gr...
please help me about calculation the Limiting Reagent and
theoritical yield of this reaction with grams in this
compounds and thank you ..
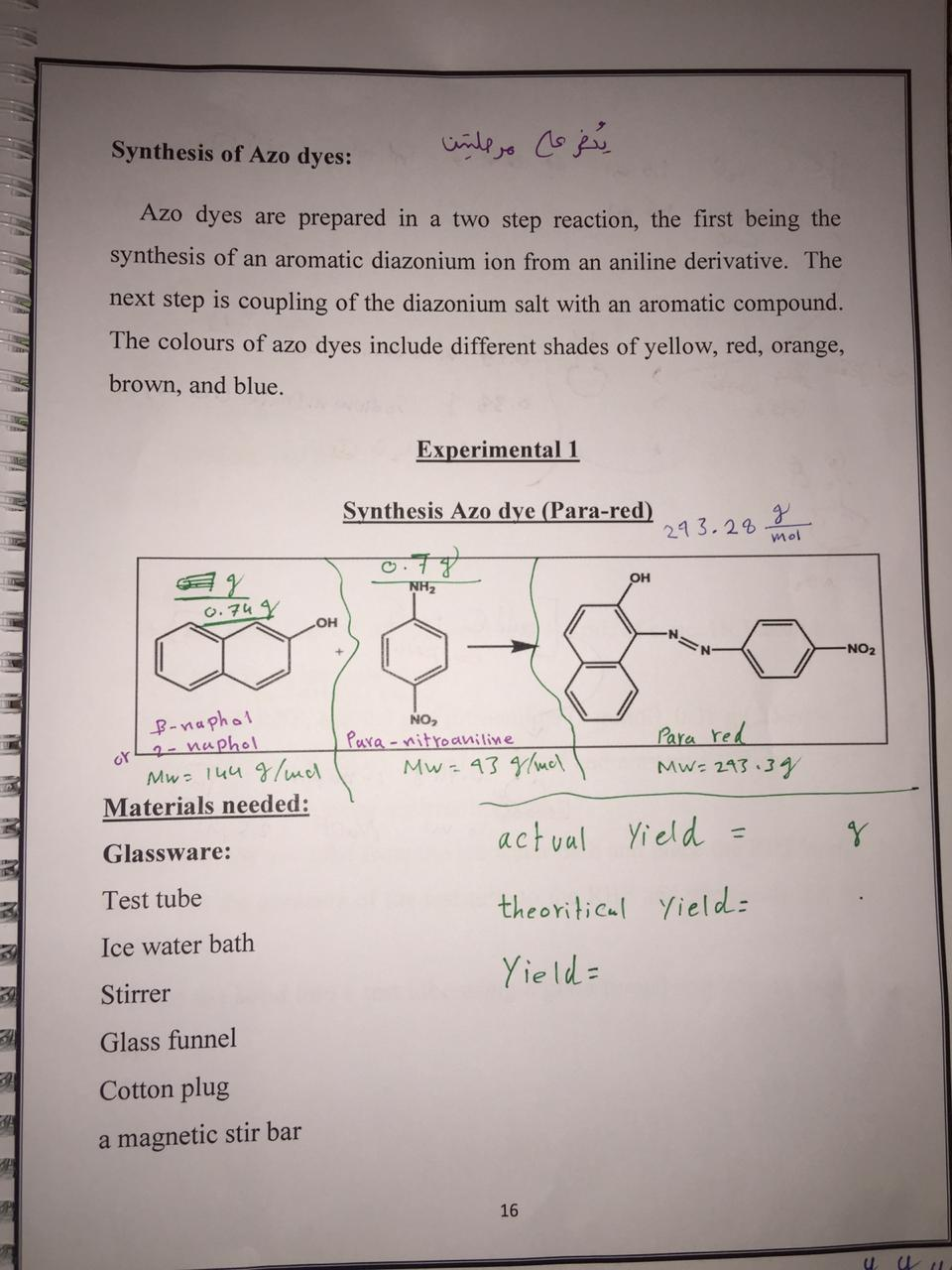
Synthesis of Azo dyes: يض عا مرملين Azo dyes are prepared in a two step reaction, the first being the synthesis of an aromatic diazonium ion from an aniline derivative. The next step is coupling of the diazonium salt with an aromatic compound. The colours of azo dyes include different shades of yellow, red, orange, brown, and blue. Experimental 1 Synthesis Azo dye (Para-red) 43.299 уи о 0.74 g OH) NO2 B-naphal - nuphol or NO, Para-nitroaniline Mw=43 g/mol. para red Mw=243.39 "Mw=144 g/ und Materials needed: Glassware: Test tube Ice water bath actual Yield = theoritical Yield: Yield= Stirrer Glass funnel Cotton plug a magnetic stir bar U
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
