Home /
Answered Questions /
Other /
4-explain-why-nonpolar-molecules-usually-have-much-lower-surface-tension-than-polar-molecules-1-usin-aw410
(Solved): 4. Explain Why Nonpolar Molecules Usually Have Much Lower Surface Tension Than Polar Molecules. 1. U...

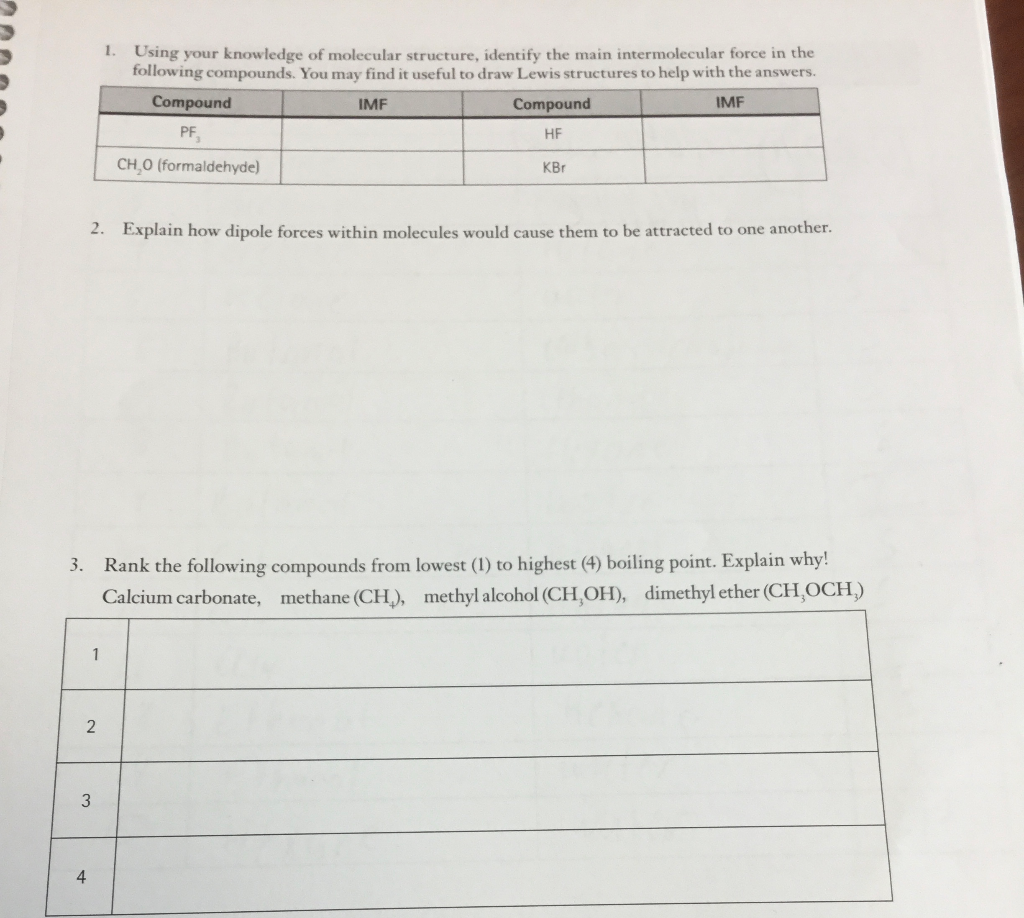
4. Explain why nonpolar molecules usually have much lower surface tension than polar molecules.
1. Using your knowledge of molecular structure, identify the main intermolecular force in the following compounds. You may find it useful to draw Lewis structures to help with the answers. Compound IMF Compound IMF HF PF CH,O (formaldehyde) KBr 2. Explain how dipole forces within molecules would cause them to be attracted to one another. 3. Rank the following compounds from lowest (1) to highest (4) boiling point. Explain why! Calcium carbonate, methane (CH), methyl alcohol (CH,OH), dimethyl ether (CH OCH) 3
We have an Answer from Expert
View Expert Answer
Expert Answer
We have an Answer from Expert
Buy This Answer $6
Buy This Answer $6
-- OR --
Subscribe To View Unlimited Answers
Subscribe $20 / Month
Subscribe $20 / Month
